
Great Ape Heart Project

For the Spring 2023 Hot Topics in Primate Welfare feature, we interviewed Dr. Marietta Danforth and Dr. Hayley Murphy of the Great Ape Heart Project (GAHP). Dr. Danforth was the project manager since the program’s inception in 2010 and took over as project director in 2021 when the project moved to the Detroit Zoological Society / Detroit Zoo. She graduated from Emory University with a B.S. in Anthropology and received her Ph.D. in Evolutionary Psychology from the University of St. Andrews. She oversees the daily operations of the project, including managing the cardiac review process, database submissions, and producing training materials and other support resources for the management of great apes in captivity. Dr. Murphy is the founder and director emeritus of the Great Ape Heart Project. She has a DVM from Cornell University and has been working in zoos since 1995. Before establishing the GAHP, Dr. Murphy was collaborating with several cardiac specialists and veterinarians to treat cardiovascular disease (CVD) cases in apes in human care.
The GAHP was established to investigate and understand CVD in great apes. CVD is a major cause of mortality for the four great ape genera managed in captivity, which includes western lowland gorillas (Gorilla gorilla gorilla), orangutans (Pongo pygmaeus, P. abelii, and P. hybrids), chimpanzees (Pan troglodytes) and bonobos (Pan paniscus). One of the main challenges in treating CVD in captive apes has been defining CVD and its causes. The project aims to improve collaboration and communication between institutions that manage apes in human care and provide resources and expertise regarding CVD to zoos, sanctuaries, and research facilities.
What are the goals of the Great Ape Heart Project?
I truly believe that the success of the Great Ape Heart Project has been because we have always focused on two interconnected goals at once – (1) providing clinical feedback for cardiac exam submissions to help care for great apes in real-time, which is based on the years of experience among our GAHP team members, and (2) creating standardized cardiac health monitoring protocols that, in turn, allow the GAHP to collect measurable, long-term trends based on the cardiac health data submitted to our database.
Ape caregivers are often asked to contribute to research projects that don’t directly affect or benefit their apes. This is a project where you get individualized feedback and support when needed. As Hayley has said so well over the years, we don’t want each zoo to have to reinvent the wheel to care for their apes. By creating the GAHP network of cardiologists, veterinarians, pathologists, anesthesiologists, nutritionists, ape care staff, and Species Survival Plan experts, we have a model for collectively studying cardiovascular diseases in great apes and for managing these conditions within the zoo community.
What were the motivations for developing the Great Ape Heart Project?
By the late 2000’s, the Species Survival Plan (SSP) veterinary advisors for the Association of Zoos and Aquariums (AZA) were aware that cardiovascular disease was the leading cause of death for great apes in managed care. The Gorilla and Bonobo SSP vet advisors were independently collecting cardiac ultrasounds (echocardiograms) in their respective databases, but it was clear that these efforts were overlapping and would greatly benefit from the inclusion of data from orangutans and chimpanzees. Based on her gorilla database efforts, Hayley led the initiative to apply for grant funding through the Institute of Museum and Library Services (IMLS). Through the first 2010 IMLS grant, it was possible to establish a formal Great Ape Heart Project and fund a full-time project manager. The overall motivation for establishing the GAHP was to create a scientifically-based foundation on which we could better understand and care for great apes.
How prevalent is cardiovascular disease (CVD) in captive ape populations? Are there differences in prevalence between species, sex, age, etc.?
Mortality reviews for great apes in North American AZA institutions all find cardiovascular disease to be a leading cause of death, ranging from 29-77% of subadult and adult apes depending on the species (orangutans, gorillas, bonobos, and chimpanzees). There are differences not only in the types of heart disease seen (e.g., left ventricular hypertrophy, aortic dissections, arrhythmias), but also in the age at which the disease presents. Apes in captivity live much longer than their counterparts in the wild (20+ years longer in some cases). As in humans, we commonly see heart disease in geriatric individuals (35+ years), but we also see cases in subadult apes. These differences in the age of disease onset may in part be explained by genetics, diet, behavior, etc. but is still something we are actively researching.

Does cardiovascular disease occur in free-ranging apes?
Yes, however, there are few published articles on heart disease in free-ranging apes. With the exception of Mountain gorillas, it is uncommon for great apes in the wild to receive regular clinical exams. Echocardiography is the most common modality for assessing cardiac health in great apes, but in the absence of it, we rely on postmortem findings. Unfortunately, the ability to perform a cardiac necropsy on a wild ape is extremely limited as well.
How does cardiovascular disease in apes compare to cardiovascular disease in humans? What are the clinical presentations of cardiovascular disease in apes?
Many of the same types of cardiovascular disease seen in humans can be seen in apes, which is why we rely so heavily on the expertise of MD advisors, who have now become subject matter experts on CVD in apes. Interestingly, atherosclerosis which can be a contributing factor in the development of CVD in humans is usually only incidental in apes with CVD.
The clinical presentation of CVD in apes can be extremely variable. Subtle signs are often the first indicators that CVD may present; reduced appetite, weight loss, reduced activity or lethargy, and importantly, social withdrawal or isolation, avoidance of antagonistic or aggressive interactions with conspecifics, and loss of social ranking. We may see signs of overt cardiovascular system dysfunction such as syncopal episodes from arrhythmias, signs of multisystemic failure from decompensated congestive heart failure, cardiac arrest during times of increased stress or activity from end-stage cardiomyopathy, thromboembolic events, or sudden death from aortic dissections. At times, CVD may be detected on opportunistic exams from changes in heart dimension on imaging, abnormalities on the ECG, the presence of hypertension, or changes to the heart structure and function on an echocardiogram.
Does hypertension in apes play a role in cardiovascular disease?
One of the most common forms of cardiac disease we see among great apes is Left Ventricular Hypertrophy (LVH) which can be a result of undetected/untreated hypertension. One of the challenges we face is obtaining consistent and reliable blood pressure in great apes.For humans, we get our blood pressure taken at practically every office visit with a doctor, but great apestypically get their blood pressure taken during an anesthetized exam, which can be as infrequent as every two years or longer, depending on the individual. Furthermore, anesthesia affects blood pressure, and, depending on the anesthetic protocol used and the time at which blood pressure is recorded, you can find a great deal of variation in blood pressure readings.
So how do you take blood pressure from an ape more regularly without the use of anesthesia? An arm cuff reading (like we do in humans) is the most ideal option for obtaining an accurate blood pressure measurement; however, apes are so incredibly strong that there’s a lot of extra equipment that needs to be used to safely train them to volunteer for blood pressure readings. You can learn more on the GAHP’s website about the “Tough Cuff” that is used for this sort of training. It is also possible to use finger cuffs so that an ape can present his or her fingers through the mesh of their home area, with little or no need for cage mesh modification. However, the farther you get away from the heart, like down to the fingers, the more sensitive the reading is to movement, so it takes time to properly train an ape to sit still in the correct position. You can read more about the GAHP’s finger blood pressure pilot project with bonobos in the recent special issue of AJP!
What are some of the challenges of performing research in the zoo setting?
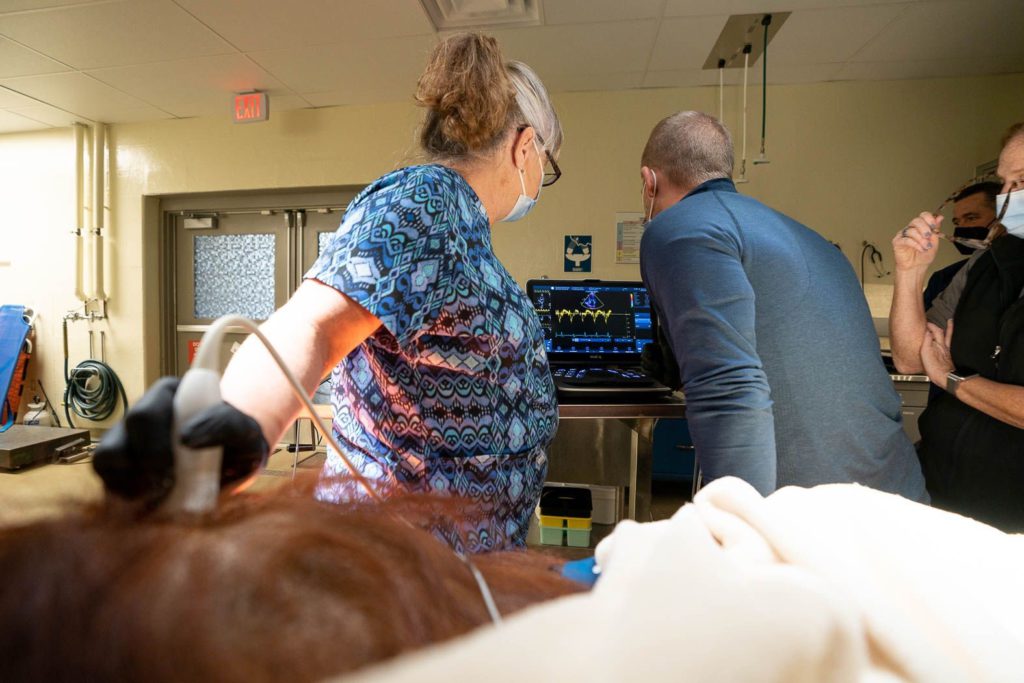
I find one of the hardest parts of my job is switching “hats” depending on the audience I am working with.In order to do the research that we do, we need the support of keepers and vet staff, and the involvement of specialists such as cardiologists (human and veterinary), pathologists, geneticists, nutritionists, etc. Each of these roles has a different perspective and focus, which makes it important to communicate the big picture as much as possible.
For example, when an ape receives an anesthetized echocardiogram, it is often done by a volunteer sonographer that works with human patients. If they are unfamiliar with great apes, they likely do not know that apes have air sacs that can interfere with getting good images. The zoo veterinarian might not know to ask for the file type “DICOM” to be saved and exported by the sonographer so that the images can be measured and reviewed by a GAHP cardiac advisor. Or, maybe they do have the huge DICOM files to send, but their zoo’s IT department blocks file-sharing sites like Dropbox. Each zoo has a different setup, so communication is essential for coordinating and sharing information.
What are the challenges of echocardiography in apes?
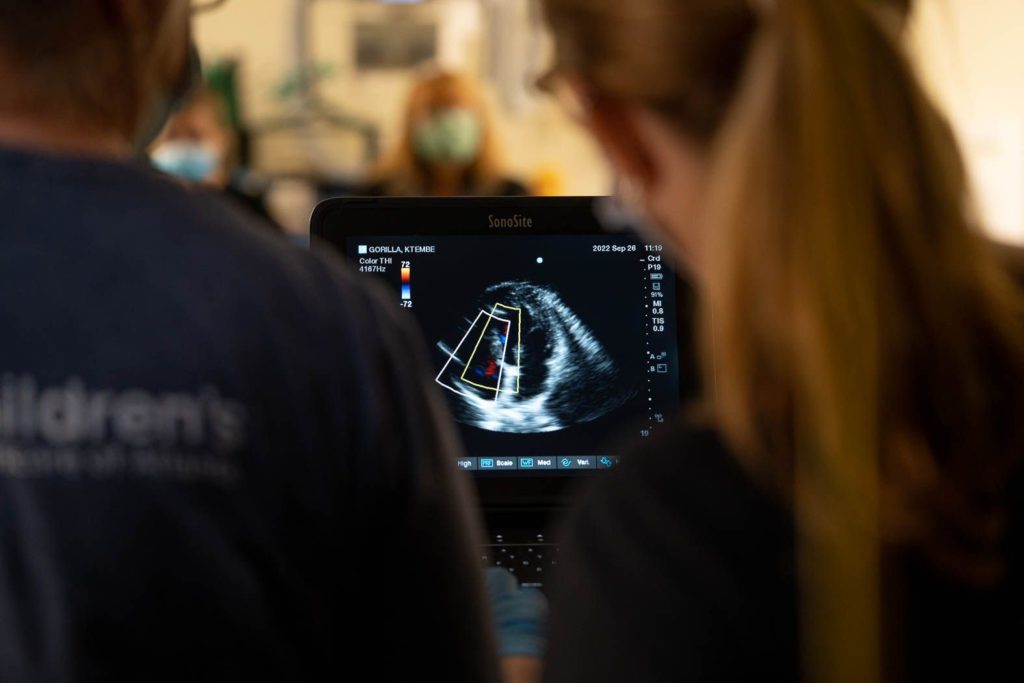
The GAHP wrote a guidelines paper for echocardiography in great apes (Boyd et al. 2019) to help communicate challenges associated with imaging great apes.
If it’s an anesthetized exam, the anesthetic protocol, particularly if alpha-2 agonists are used, may interfere with the interpretation of the heart’s true function. The quality of the machine and probe will determine if you can obtain quality images in great apes. Depending on the size and shape of the ape, certain views can be obstructed by the air sac (e.g., adult male orangutans), or hard to visualize due to the depth of the heart within the chest (e.g., older gorillas or chimpanzees with more pointed chests).
If it’s an “awake” exam (i.e., an unanesthetized exam) where the ape is trained to present his or her chest, challenges include desensitizing the ape to the feeling of ultrasound gel (more is better for image clarity), getting the ape to sit still long enough to obtain enough images for a complete exam, and of course, convincing the ape not to destroy the $8-12K probe!
What are the requirements for institutions to participate in the Great Ape Heart Project?
The GAHP has historically focused on cardiac ultrasounds, so contributing these exams to the database is the most common way in which we think of participating in the GAHP. We may not be able to provide a diagnostic report in every case, but we’ll do our best to provide feedback, even if it’s advice for improving future exam quality.
We welcome inquiries and cardiac health data from zoos, sanctuaries, and primate research centers worldwide.
What kind of technology and services can institutions access through the Great Ape Heart Project?
The sonographers and cardiologists for our project assist a variety of institutions in performing their anesthetized exams. Since they typically take leave from work for these exams, we ask that institutions reach out a few months in advance to check their availability and whether they need to bring the GAHP ultrasound machine. They do not charge zoos a fee, but travel costs must be covered by the institution.
GAHP pathologists, Dr. Karen Terio (University of Illinois Urbana) and Dr. Gisela Martinez Romero (Tufts University) accept hearts for standard and advanced cardiac necropsies ($300-$600). They provide full reports back to the submitting institution. The GAHP is particularly interested in heart submissions for great apes where we also have good-quality serial echoes in our database. In those cases, zoos have also received in-depth case reviews by the GAHP team.