
Celebrating the ASP 2024 Primate Welfare Award Winners
This Hot Topics in Primate Welfare, we are featuring the work of the two ASP 2024 Primate Welfare Award winners. Jaclyn Allen received the award for her project “The use of guanfacine to mediate anxiety-related reactivity and reduce associated agonistic behavior in breeding male pigtail macaques (Macaca nemestrina).” Jose Tello received the award for his project “Challenges and outcomes of transitioning to fruit-free diets in primates.”
Ms. Jaclyn Allen, BS (right) currently works at Johns Hopkins University’s primate breeding facility as the Manager of Primate Behavior and Colony Management. Jaclyn studied at Towson University earning a Bachelor’s of Science in Biology, specializing in Zoology and Ecology. In her current role, she directs the behavior, breeding, enrichment, and animal training programs for the facility. Promoting positive welfare and species-typical behaviors for the macaques in her care is a top priority. When a case of severe agonism jeopardized the welfare of group-housed pigtail macaques (Macaca nemestrina), Jaclyn and her colleagues studied an innovative use of guanfacine to treat social reactivity. Read below for an interview with Ms. Allen regarding this project and check out the recent publication based on this work here.


Mr. Jose Tello, MSc (left) works as the Head of the Nutrition Department at the Parque Zoologico Nacional la Aurora in Guatemala. Since 2022, he has overseen animal nutrition at the zoo, working to incorporate fruit-free diets for various species of primates. This includes making dietary changes according to species-specific nutritional requirements and recording animal responses to those changes. The research project featured here focuses on the challenges of making the transition to fruit-free diets in zoos, including challenges with keepers, curators, and veterinarians, while obtaining positive outcomes on animal welfare, such as improvements in body and fecal condition, reduction of aggression, and enhanced responses to training in different primate species. Watch a video presentation of Mr. Tello’s project below.
Jose Tello’s Project Presentation
Interview with Jaclyn Allen
Your title at Johns Hopkins University is Manager of Primate Behavior and Colony Management. What is entailed in behavioral and colony management?
I direct the behavior, breeding, enrichment, and animal training programs for the Johns Hopkins University (JHU) primate breeding facility, where we have two colonies of non-human primates: pigtail macaques (Macaca nemestrina) and rhesus macaques (Macaca mulatta). The macaques live in social groups, housed in indoor-outdoor enclosures, and it is my priority to promote their species typical behavior and ensure their welfare.
What kinds of behavioral issues are most common in captive breeding colonies? What types of interventions are utilized?
Fortunately, due to our behavioral management program, we do not have many behavioral concerns. The main issue we face with group housed macaques is social wounding. Agonism is a species-typical and integral part of macaque societies, used to maintain complex relationships and hierarchies. However, in severe cases, behavioral management strategies and therapeutic intervention are often required to maintain compatible social groups and promote positive welfare. This is important in a breeding colony to ensure successful reproductive outcomes. At times, we expect to see higher rates of agonism, such as during rhesus breeding season, during breeding interactions, or during social introductions and group formations. While managing these scenarios, we implement strategies to mitigate aggression and oftentimes implement strategies proactively to prevent escalation. Common interventions include giving the animals more housing space, changing housing location, changing style of housing, increasing enrichment plan frequency, implementing visual barriers, administering therapeutic intervention, and, in drastic scenarios, removing group member(s) and moving them to other social groups. Additional behavioral concerns we may encounter with our breeding colonies are resource guarding, social overgrooming, and infant neglect.
What is the general process for identifying behavioral issues, deciding on a particular intervention, and then assessing the effectiveness of that intervention?
Knowing the natural history of the species and their social repertoire is essential. When you understand species-typical behaviors, it is easy to identify behavioral concerns. When an issue is identified, observations are performed by the Behavior Management Team to investigate what is triggering the concerning behavior. We then work closely with our veterinary team and our animal care team to develop a treatment plan. Once a plan is implemented, the individual/group is monitored daily with data collected as needed to analyze a change in behavior.
What led your team to this project in particular?
An adult male pigtail macaque in our breeding colony had a history of directing aggression and wounding to all his group mates, often necessitating veterinary treatment. While wounding events were not frequent, they were fairly severe. Numerous behavioral management interventions had already been implemented; however, overt aggression and wounding continued, even while he was maintained on fluoxetine. As animal welfare is always our highest priority, we were motivated to explore new treatment options.

Why did you decide to try guanfacine as an intervention? What are the risks and benefits to guanfacine administration?
Guanfacine is an ADHD drug that has been shown to reduce impulsivity in human patients as well as in animal models with self-injurious behavior (SIB), as reported by JHU researchers Freeman and colleagues in 2015. Given the pathways that guanfacine mediates, we theorized it may represent a viable treatment for our male, who seemed to show aggressive behavior when he was anxious about external stressors. To our knowledge, it had never been used to treat social reactivity in pigtail macaques previously.
The main risk associated with guanfacine is animal compliance. Due to a high dose and very bitter taste, some animals may be reluctant to take it. This is why the legacy effect, wherein the effect of the drug continues even following discontinuation, is so beneficial. Freeman et al. (2015) found guanfacine had an apparent legacy effect for 4 weeks following the cessation of treatment for SIB. If a dose is occasionally refused, the therapy is still effective.
How was this medication administration implemented (from both an experimental design and daily logistics perspective)?
Guanfacine (10mg by mouth once per day) was onboarded while simultaneously weaning off of the fluoxetine (20mg by mouth once per day). For our evaluation, behavioral data was collected through five study phases over the course of a year, including a washout phase following guanfacine treatment to test the legacy effect. In short, we found that the male pigtail showed significantly fewer events of wounding and aggressive behavior when maintained on guanfacine long-term (see figure below). However, when we withdrew the drug, an increase in agonistic behavior was observed, so guanfacine administration was resumed.
In terms of daily logistics, we anticipated a challenge with compliance going into this study due to the bitter taste of guanfacine. To identify an ideal vehicle to administer the drug, we performed preference testing with high value treats (e.g., Skittles, Starbursts, etc.) prior to guanfacine administration, noting not only which treat was preferred, but also which flavors. However, once guanfacine was mixed with those treats, he would not touch them. It took a lot of patience and creativity to find a vehicle that was accepted …seventh time was the charm! Since we found his preferred treat (grapes mixed in strawberry jelly), guanfacine has been administered daily with 100% compliance. He has been reliable on treatment since 2022.
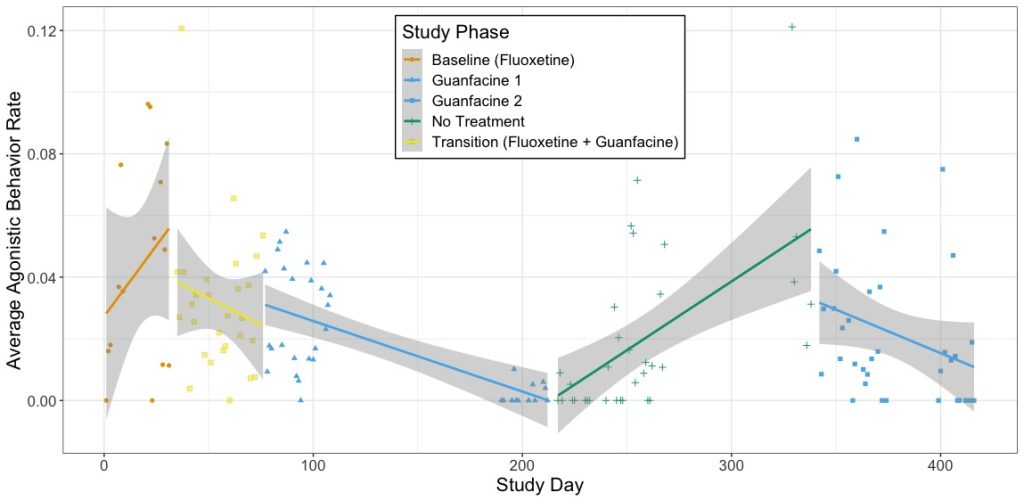
Figure appearing in the publication of these data (found here). Average rates of agonistic behavior shown by the focal male pigtail macaque by study day across each of the study phases and treatment types. The line shows the linear relationship between study day and rate of agonistic behavior observed per day, and the shaded areas show confidence intervals for the linear models.
What were the outcomes? In what ways has guanfacine treatment been successful?
The behavior categories we investigated were agonism, anxiety-related behaviors, and affiliation. We wanted to assess the efficacy in reducing aggression and the interplay between reactivity and agonism, as well as ensure there would not be a sedation effect that could negatively impact breeding outcomes. We found that guanfacine significantly reduced rates of agonistic and anxiety-related behaviors without suppressing overall social behaviors, proving to be a more suitable treatment. With this therapy, the male remains a productive breeder in social groups.
Following the success of this male’s case, we effectively treated two additional pigtail macaques with guanfacine. First, an adult female in a research setting showed a complete cessation of cagemate-directed wounding once maintained on guanfacine. Last year, we began treatment with a second breeding male pigtail macaque who, like the first male, showed intense but rare agonistic behavior toward his groupmates. Now on guanfacine, he has exhibited a marked reduction in wounding events and wounding severity. A note on logistics of these cases: each pigtail had a unique vehicle preference to receive guanfacine. The female preferred Starbursts and the second male preferred Laffy Taffy. Keep in mind, what works for one animal may not work for another.
What are the implications and lessons that should be taken from these findings?
We found guanfacine to be a viable treatment for social reactivity. With detailed behavioral observations prior to the administration of guanfacine, we were able to determine that the male was not anxious overall, but rather, overreacted to environmental stressors. The collaboration of our veterinary and behavioral management teams was key in identifying both the driver of the male’s aggressive behavior and an appropriate therapy (guanfacine). Having a unified, well-rounded front when approaching behavioral cases is imperative for success and the wellbeing of our monkeys.
Discovering a solution brought forth multidimensional enhancements to welfare. First and foremost, the welfare of the male’s groupmates increased with the decrease in both frequency and severity of wounding received. Second, the welfare of the case study male was improved, as he was able to remain in group and continue breeding. Without guanfacine treatment, his social housing future was uncertain. Next is a positive impact that I refer to as “program welfare.” We were able to maintain our breeding program productivity and diversity, as we retained the male’s genes and offspring in our colony. Also important, this positive outcome improved staff welfare. Start to finish, this case was a difficult for us. In a field where compassion fatigue and burnout are high, improved welfare for the animals is also improved welfare for the humans.
Are there any unanswered questions remaining from this study? Are there additional experiments or interventions planned?
We do not have any follow up studies involving this pigtail male planned at this time. Guanfacine, in combination with housing strategies, has allowed us to successfully manage this case study male. Since this study, he has been rotated into a new breeding group and remains stable on his treatment plan.
However, within the past month, we began treating an adult rhesus macaque male with guanfacine. Just as with pigtails, to our knowledge, guanfacine has not been used to treat social reactivity in rhesus macaques. This male is one of our research retiree residents who, with his adult female companion, acts as an ‘uncle’ to a juvenile group. Like the pigtail males, he also directed aggression towards his juvenile groupmates in response to external triggers. While it is too early for us to comment on the efficacy of the treatment, it looks promising so far, and he has shown excellent compliance with daily treatment. To learn more about our retiree program, which includes the rhesus “uncle” male, please read this recent publication: https://www.mdpi.com/2306-7381/11/11/560
In what ways might these findings be applied more broadly (e.g., to other captive settings, situations, species, and to primate welfare in general)?
As nonhuman primates are socially housed in a variety of settings (e.g., research facilities, zoos, sanctuaries, and breeding centers), this innovative application of guanfacine may be beneficial in a wide variety of environments. There is potential for guanfacine to be used not only in a variety of species, but also a variety of situations that cause social anxiety. I am particularly curious about its application in zoo-housed primates that are reactive to visitor presence, which may represent an unpredictable external stressor.